There’s no shortage of research efforts looking for ways to stop, or at least slow down, the novel coronavirus. Of course, those strategies involving cutting edge techniques, including CRISPR gene editing, tend to get most of the attention. If it’s new, it must better, after all.
But what if we could reach back 100 years for a solution? That’s essentially what we’ve done with the recent decision by the US Food and Drug Administration to authorize the emergency use of an old technique — convalescent plasma — for patients severely ill with COVID-19. The idea is that plasma from people who have recovered can transfer protective antibodies to a still-sick recipient. Donors must have been symptom-free for 14 days with a negative test or for 28 days without one.
“We think it shows promise, and we’re going to be starting that this week,” said New York governor Andrew Cuomo just before the announcement.
Natural antibody cocktails
The rich history of convalescent plasma meanders through the plagues of the twentieth century. Hearing about it in the context of COVID awakened memories of receiving a similar treatment, in the 1960s.
Every fall, I got a shot of something with a funny name: gamma globulin. I thought it had something to do with goblins, since Halloween was coming. The mixture of antibodies (aka immunoglobulins), from healthy donors, might protect me from whatever infectious diseases was coming that winter. My uncle, our family doctor, gave the shots.
The hazy memory sent me to my old medical books. And there in “Modern Medical Discoveries,” by Irmengarde Eberle and published in 1948, I found “A weapon from our own blood stream.”
The book describes the separation of blood into components during World War II, so that when blood supplies ran short, wounded soldiers could receive plasma. Edwin Cohn from Harvard Medical School pioneered separating gamma globulin, the “part of blood in which are found all the disease resisters, or antibodies, which have developed in a person’s blood when he has had certain of the infectious diseases,” wrote Eberle.
The wartime gamma globulin work was secret, but it spiked interest in antibodies. In 1942 Linus Pauling wrote to Dr. Cohn, asking for samples to create “artificial antibodies.” Blood was swimming in “resisters” against the scourges of the time, he wrote: diphtheria, mumps, pertussis, and scarlet fever. Gamma globulin was given to thousands of military personnel, and years later, found its way to my uncle and my arm.
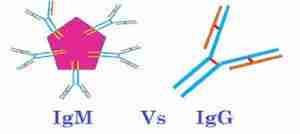
Convalescent plasma teems with the abundant gamma globulins (IgG) made once an infection gets underway, plus antibodies called IgM that are made early on. Some antibody types “neutralize” the pathogen, dismantling it, stripping off surface proteins, preventing reproduction, stimulating release of immune system chemicals, and clumping them, making viruses and bacteria more visible to the immune system.
Immunology 101
The antibody response is called “humoral” because antibodies are carried in the blood, a fluid or “humor.” T cells and their secreted proteins make up the other arm of the immune response, called “cellular.”
A natural antibody response is “polyclonal,” or multifaceted. Big, blobby plasma cells churn out antibodies that fit, like keys in locks, specific parts of a pathogen’s surface. An individual plasma cell releases only one type of antibody, at a rate of several thousand a second once stimulated. Altogether the collection of plasma cells releases a flood of antibodies, like attacking an intruder by whacking him on the head, tripping him, and kicking his shins, all at once.
Plasma is the straw-colored liquid that remains after cells and fragments are removed from blood. Taking out the clotting factors leaves serum.
Antibodies are proteins built of Y-shaped subunits: IgG has one, IgM five. The tips of the antibody’s arms (epitopes) bind parts of a pathogen (antigens). Neutralizing antibodies can be given in convalescent serum or plasma without even knowing what the germ is, which may explain why the technique has been used for so long.
Convalescent plasma is termed “passive immunotherapy” because the recipient gets the antibodies and not the plasma cells that produce them. So the effect won’t last, but may be enough to save someone.
To donate plasma, blood is typed (ABO and Rh) and screened for viruses. Then a technique called plasmapheresis removes antibodies from plasma and returns the cells to the person, replacing the plasma with saline. It takes about 90 minutes.
A donor can give plasma more frequently, and more per session, than donating whole blood. And if the donor lives near the recipient, chances are that both individuals were infected with the same version of a pathogen.
The ‘childhood diseases’
The first attempts that led to convalescent plasma were done in animals, using serum from rabbits and horses, in the 1880s.
In 1894, Emil Behring, who won the Nobel Prize in Physiology or Medicine in 1901, published findings on 220 children with diphtheria, a bacterial infection. Serum given within two days of diagnosis was 100% successful in treating the disease, but the rate dropped in children who had been sick longer.
In 1897, German surgeon Leopold Weissbecker treated 5 children who had scarlet fever, another bacterial infection. This time it didn’t work.
A superstar in convalescent plasma lore was physician/scientist Abraham Zingher, who worked for the New York City Department of Health. In 1916 he and co-worker William Park injected 48 children who’d been exposed to measles with serum from children who had recovered; 28 got low doses and 20 high.
Six low-dose kids got measles, and none of the high-dose kids did. On August 15, 1916, The New York Times reported, “Dr. Zingher, discussing his suggestion that physicians all over the city inject into the children serum made from the blood of parents , said last night he was not sure that such injections would avail, but anyway, he said, they would be harmless.”
Dr. Zingher published an account of his work on measles in 1924, and he then used serum to prevent or lessen severity of polio and scarlet fever. A strange footnote is that he was found dead sitting at his lab bench at Willard Parker Hospital at age 42 from inhaling toxic gas.
Pandemic influenza: 1918 and 2009
While Dr. Zingher was focusing on childhood diseases, the “Spanish flu” (H1N1) swept the world, killing 50 to 100 million. To treat the acute respiratory distress syndrome and multi-organ failure that killed patients, desperate doctors turned to convalescent plasma.
Eight studies conducted between 1918 and 1925 followed the fates of 336 patients who received plasma and 1,219 who didn’t. The fatality rate was 16% for those who did and 37% for those who didn’t, and the earlier the treatment, the more effective it was.
In 2009, a novel influenza strain, also H1N1, arose in Asia that resembled the 1918 killer. Researchers in the US military investigated use of convalescent plasma saved from survivors of the 1918 pandemic, but supplies were sparse.
Then investigators from Hong Kong tried plasma from people who’d survived the novel 2009 influenza. They recruited 93 patients requiring intensive care, 20 of whom volunteered to receive plasma.
Mortality was 20% among the treated patients compared to 54.8% among the patients declining plasma. The treated patients also had lower viral loads and cytokine responses (a cytokine “storm” is what often kills, as the immune system goes into warp drive).
Fortunately the 2009 influenza pandemic dampened naturally into a mild seasonal illness. But epidemiologists knew it could happen again. So the team from Hong Kong evaluated the feasibility of collecting plasma from many people.
Results were disappointing. Of 9,101 people who’d recovered, 301 donated plasma and 379 gave blood, amounting to only 276 liters of convalescent plasma.
A parade of viral diseases: from SARS to Ebola
After antibiotics came along in the 1940s, which treat bacterial infections, convalescent plasma turned to viral diseases: mumps in 1944, a viral rash called pityriasis rosea in 1957, Lassa fever in 1984 and Argentinian hemorrhagic fever in 1985. The list also included chickenpox, parvovirus B19, rabies, hepatitis B, cytomegalovirus, respiratory syncytial virus and polio.
Doctors didn’t know exactly what they were putting into their patients, but it seemed to work. And the numbers started to build.
Key evidence came from Argentinian hemorrhagic fever, caused by Junin virus. Researchers tracked 23 annual epidemics spanning 1959 to 1983, involving 4,433 patients. Mortality among patients receiving standard treatment was 42.85%, but for those who received convalescent plasma, it was only 3.29%.
With those stats, it made sense to try convalescent plasma on SARS.
A study of 80 patients in Hong Kong from the year SARS emerged, 2003, showed that 58.3% of patients given convalescent plasma were discharged before day 14 compared to 15.6% of others. The plasma was even more helpful if given before exposed individuals tested positive. Yet one research team from China working on COVID-19 calls convalescent plasma to treat SARS “a last resort,” but acknowledge that it seemed to have worked.
Researchers were unnerved by the lack of neat, randomized, controlled clinical trials. But medical ethics and a time of desperation intervened.
When Ebola broke out in Guinea in 2016, the World Health Organization prioritized testing convalescent plasma, pointing to success in 1995 in Zaire and in 2014 in Liberia. In Guinea, 99 patients received two doses of convalescent plasma. The controls were 418 patients who’d been treated at the same facility over the past 5 months, but who hadn’t received plasma.
Those getting convalescent plasma recovered faster from Ebola and 31% died compared to 38%. The researchers didn’t consider this significant, but another report showed that it can take nine months or longer for neutralizing antibodies against Ebola virus to appear. So it might have worked.
The Ebola studies suggested that plasma donations must match the timetable of the natural antibody response to a particular pathogen. And some pathogens, like MERS, which struck Saudi Arabia in 2016, might not elicit a strong enough antibody response for plasma donations to be possible.
COVID-19 and convalescent plasma
COVID-19 and the puzzling virus behind it have only been on our radar a few months. Until clinical trials can more objectively evaluate convalescent plasma, limited case series must suffice. The Infectious Diseases Society of America (IDSA) considered two recent case series, totaling 15 patients.
The first report, from Kai Duan and colleagues at the Wuhan Institute of Virology, looked at 10 patients given one dose of plasma and anti-viral medication. The patients developed high levels of neutralizing antibodies and improved within 3 days, with no detectable virus in the blood by day 7. Their lungs cleared.
The second case series, from Chenguang Shen and co-workers at Southern University of Science and Technology in Shenzhen, China, followed 5 critically ill patients who were taking anti-virals and who improved rapidly. But an accompanying editorial claimed “it is not possible to determine the true clinical effect of this intervention or whether patients might have recovered without this therapy.”
The IDSA concluded that the patients in the two studies differed too much – in stage and severity of disease, other treatments, and baseline characteristics – to be compared. So even though the convalescent plasma didn’t do any harm, IDSA advised it be given only “in the context of a clinical trial.”
Are monoclonal antibodies the answer?
The flow of evidence supporting use of convalescent plasma continues, a few cases at a time, hopefully leading to a torrent of supportive data that will come from the large-scale clinical trials getting underway. ClinicalTrials.gov currently lists 45 entries for “convalescent plasma for COVID-19,” from 16 nations, all bearing the blue banner “NEW.”
#45 grabbed my attention. Researchers from Hospices Civils de Lyon in France and Eurobio Scientific are harnessing monoclonal antibody (MAb) technology to select and mass-produce the exact antibodies needed to neutralize the virus, rather than the soup that is convalescent plasma. MAb technology, around since 1975, has been used to diagnose everything from cancer to pregnancy to turf grass disease.
In the clinical trial, B cells (which mature into plasma cells) from the blood of five recovered patients are fused with cells that divide continuously. Because a B cell makes one type of antibody, the “immortalized” cell secretes huge amounts of a single, or “monoclonal,” antibody type – chosen because it vanquishes the virus. The pure antibodies will be given to five very sick patients.
Researchers at Utrecht University in the Netherlands have isolated a neutralizing antibody a different way – from collections of antibodies they already had from the original SARS coronavirus, from 2003. In fact it fights both coronaviruses. “This cross-neutralizing feature of the antibody suggests it may have potential in mitigation of diseases caused by future-emerging related coronaviruses,” said Berend-Jan Bosch, co-author of the paper in Nature Communications describing the work.
A monoclonal antibody-based biologic will bring precision medicine to the mixed bag of possibilities that is the antique technique of convalescent plasma. Tapping into the exquisite specificity of antibodies takes advantage of the evolution of the human immune response.
Ricki Lewis is the GLP’s senior contributing writer focusing on gene therapy and gene editing. She has a PhD in genetics and is a genetic counselor, science writer and author of The Forever Fix: Gene Therapy and the Boy Who Saved It, the only popular book about gene therapy. BIO. Follow her at her website or Twitter @rickilewis